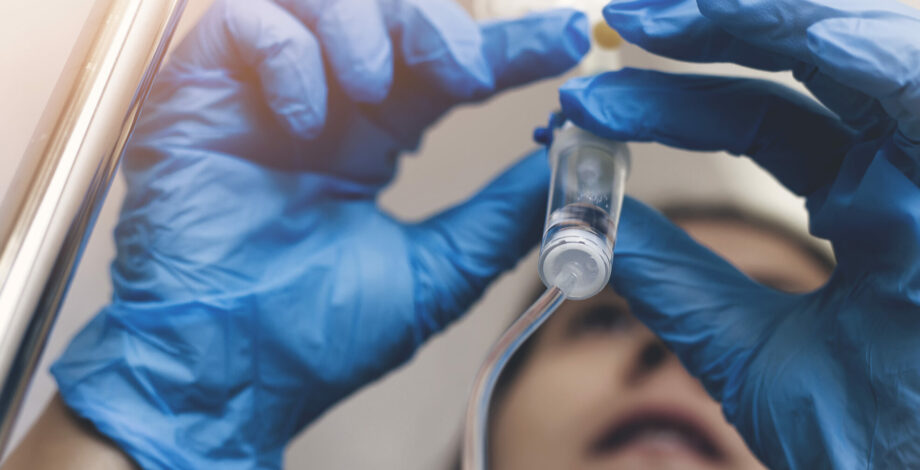
November 11, 2022 — What’s in that IV bag besides saline and medication? As we become more aware of the harms of chemicals embedded in plastics and other materials, consumers — including patients — are demanding a higher standard.
Research suggests that the presence of certain chemicals in some healthcare equipment can compromise the benefit of the treatment the equipment enables. For example, the presence of chemicals in intravenous equipment could reduce the effectiveness of the medication being administered and alter hormonal function in patients.
In response, the nonprofit organization Clean Production Action recently created a process to certify health-care products that do not contain specific chemicals such as polyfluoroalkyl substances (PFAS) that are associated with cancer, altered brain development, endocrine disruption and other health harms.
To earn certification for a product under the new GreenScreen Certified Standard for Medical Supplies & Devices, health-care product manufacturers must follow basic principles of green chemistry and prove it is formulated without disallowed chemicals through rigorous testing. They also need to recycle or otherwise address waste implications of both the product and its packaging.
Various levels of certification are available depending on the level of strictness the manufacturer wishes to pursue with respect to different chemicals. Manufacturers can use the certification to show consumers that they are conscientious about avoiding harm and to identify products that contribute to meeting consumers’ environmentally preferred purchasing (EPP) goals.
In addition to healthcare equipment, Clean Production Action also offers certifications that apply to textiles, cleaners, degreasers, firefighting foam, fabrics, furniture and food service equipment.
Related Posts
Ensia shares solutions-focused stories free of charge through our online magazine and partner media. That means audiences around the world have ready access to stories that can — and do — help them shape a better future. If you value our work, please show your support today.
Yes, I'll support Ensia!