November 17, 2014 — Sandwiched between rows of bottled chemicals and racks of drying glassware on the third floor of Harvard University’s Mallinckrodt laboratory, four thumbnail-size bits of pinstriped silicon hang from a thin metal wire like T-shirts on a clothesline. For something with the potential to transform our energy infrastructure, this technology could not look less unassuming. But in the short history of the pursuit of artificial photosynthesis — the conversion of sunlight into fuel rather than electricity — looks have all too often been deceiving.
In 2011 Daniel Nocera, then a professor at the Massachusetts Institute of Technology, made headlines around the world with something he called the “artificial leaf.” The device, a solar cell the size of a small Post-it note, promised to revolutionize solar energy.
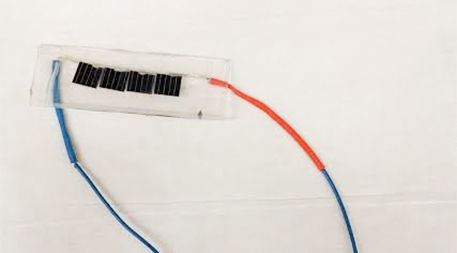
Do these unassuming bits of crystalline silicon hold the future of sunlight-to-fuel conversion? Photo by Phil McKenna.
Like conventional photovoltaics, the artificial leaf used common semiconducting materials (in this case, amorphous silicon) to absorb sunlight and emit electrons. But then it went one step further. When dipped into a beaker of water, instead of producing electricity, the leaf harnessed the electrons to break the chemical bonds of water and release hydrogen gas — a fuel that can store energy at a significantly higher density and lower cost than electricity. And it did so in a remarkably efficient way, converting 5 percent of the energy in sunlight that hit it into hydrogen fuel, compared to a 1 percent sunlight-to-chemical-energy conversion efficiency in plants.
At the time Nocera noted that water from an Olympic-size swimming pool could meet the energy needs of every person on Earth. Ratan Tata, then head of the Indian industrial conglomerate Tata Group, partnered with Nocera, saying the technology could soon bring power to billions of people in developing countries.
The hype, however, turned out to be ahead of reality. Nocera’s initial device began to degrade after just 10 hours underwater and Sun Catalytix, a company he founded, quietly shelved plans to develop a larger prototype.
Fast forward three short years. Nocera, now at Harvard University, as well as scores of other researchers around the world, are on the verge of turning the promise of artificial photosynthesis into reality.
Conquering Corrosion
Natural photosynthesis has been around for billions of years, but it’s not very efficient at turning sunlight into energy-dense chemicals we can use for fuel. “It tends to make carbohydrates — which are great for feeding us, but they aren’t very good at powering a car,” says Carl Koval, director of the Joint Center for Artificial Photosynthesis.
“Four years ago you would have said this would not be possible.” — Carl KovalFormed in 2010, JCAP is a $122 million federally funded initiative based at the California Institute of Technology. Its assignment was to develop a viable artificial photosynthesis device by 2015. The prototype had to be durable, be made from commonly available materials and convert sunlight to fuel at an efficiency of 10 percent.
“Four years ago you would have said this would not be possible,” Koval says.
One of the biggest hurdles JCAP had to overcome, and one that plagued Nocera’s initial device, was corrosion of the light-absorbing components. For artificial photosynthesis to work, semiconductors such as those found in conventional solar cells must absorb incoming light and convert it to electricity. Then a pair of catalysts, typically on opposite sides of the semiconductors, need to use that electricity to split water to produce oxygen and hydrogen. To protect the semiconductor from corrosion, the catalysts have to form a protective coating while simultaneously driving the water-splitting reactions. This, it turns out, is incredibly difficult. The coatings have to be thick enough to keep corrosion-inducing water out, but, depending on the architecture of the device, one of them typically has to be thin enough to allow sunlight to pass through.
“People were thinking we had to develop new [semiconductor] materials that are inherently stable,” Koval says. It took researchers decades to develop the semiconductors we use for conventional solar power, so having to start over with new materials would likely have derailed any near-term commercial prospects for artificial photosynthesis.
JCAP has since assembled lab-scale prototypes that have run for thousands of hours with little degradation at efficiencies exceeding 10 percent.Then, in May 2014, JCAP researchers at Caltech used a process called atomic layer deposition to form a thin protective coat of titanium dioxide over a number of common semiconducting materials. The process created an effective barrier against water, yet allowed sunlight and electrons to pass. When a small amount of nickel oxide catalyst was added on top of the titanium dioxide, the combined coating facilitated a sunlight-to-hydrogen conversion at efficiencies approaching those of conventional photovoltaics.
“When I first saw those results they sounded too good to be true,” Koval says. “It just really opens up a whole toolbox of material combinations that no one could have used before.”
JCAP has since assembled lab-scale prototypes that have run for thousands of hours with little degradation at efficiencies exceeding 10 percent.
“Now you can put this on a bench top and show people an artificial photosynthesis device that, with additional development, can be turned into a deployable technology,” Koval says.
The prospect of being able to use CO2 as an ingredient in fuel is a huge motivator.JCAP is currently seeking additional funds to solve a key related challenge: How to transform hydrogen gas produced by artificial photosynthesis into a more useful fuel. As Koval points out, even though use of hydrogen in fuel cells is growing, the biggest need is for liquid fuel to run cars, trucks, trains and planes.
To covert hydrogen to fuels such as gasoline, carbon dioxide has to be added in a high-temperature, energy-intensive process. Finding a more efficient method of conversion will likely prove challenging. The prospect of being able to use CO2 as an ingredient in fuel is a huge motivator, however. Nowhere is the potential use of CO2 more appealing than in China, now the world’s largest emitter of the greenhouse gas.
“We try to use this waste material as a useful feedstock,” says Can Li, director of the Dalian National Laboratory for Clean Energy in Dalian, China. Li heads a team of more than 50 researchers working on artificial photosynthesis and hydrogen-to-liquid-fuel conversion, one of more than half a dozen such teams around the world. “I think industry people are starting to get interested because they can make money while doing waste reduction,” he says.
Economic Challenge
In his laboratory at Harvard, Nocera alternates between pacing excitedly and staring listlessly out the window. He seems energized by recent technical achievements, yet daunted by the challenges of bringing the technology to market during the current gas and oil boom.
Since he introduced the artificial leaf in 2011, Nocera developed his own stable, oxygen-producing catalyst that, like JCAP’s encapsulating material, works well with a number of existing light-absorbing semiconductors. Issues of cell durability with the new catalyst have been worked out to the point that he doesn’t even bother testing each new device in water.
“The science is ready,” he says.
What remains a challenge is the economics. The bits hanging from a wire on his bench today are made of crystalline silicon, a semiconducting material commonly used in today’s conventional solar cells. In August, Nocera and postdoctoral fellow Casandra Cox demonstrated a 10 percent solar-to-hydrogen conversion efficiency using the material. Crystalline silicon isn’t the most efficient semiconducting material; researchers at the National Renewable Energy Laboratory in Golden, Colo., have achieved more than 16 percent sunlight-to-hydrogen conversion efficiency using gallium, indium and arsenide. But it is relatively inexpensive — and getting cheaper. With this low-cost material Nocera estimates he could produce a kilogram of hydrogen, the fuel equivalent of a gallon of gasoline, for approximately $2.
“Until you put a price on carbon, none of these technologies are going to get a foothold.” — Daniel NoceraBut even that price, which does not include the cost of the fuel cell that would be needed to convert hydrogen back to electricity, is not low enough to displace existing energy infrastructure, Nocera says.
“It’s still not good enough, because we have fracking,” he says. “Until you put a price on carbon, none of these technologies are going to get a foothold,” Nocera says.
Nevertheless, given how quickly the technology is developing and how quickly semiconducting material prices are falling, Nocera’s own lab mates may yet prevail upon him to find a way toward commercialization.
“I think it’s time to start looking at how to scale it up,” Cox says. ![]()
Ensia shares solutions-focused stories free of charge through our online magazine and partner media. That means audiences around the world have ready access to stories that can — and do — help them shape a better future. If you value our work, please show your support today.
Yes, I'll support Ensia!

With the global climate pact in place this is a must. This idea is only good after the other is done. All the research money should be going for wind and solar first, it's more accessible now.